System Services (CzeekV Pro/CzeekR)
Drug Adverse Events Information System CzeekV Pro
*ASP Service: Available immediately after you log in with your own ID
Contains adverse event report data worldwide
- Installed both FAERS and JADER databases
- Enables search and display in Japanese by data arrangement including elimination of duplicates
*FAERS (FDA’s Adverse Event Reporting System): a public database administrated by the FDA containing adverse event reports, medication error reports and product quality complaints resulting
*JADER (Japanese Adverse Drug Event Report database): a public database administrated by the PMDA containing adverse event reports.
Exploration of relationship between "drugs and adverse events" and statistical analysis
- Signal detection index values are calculated by four statistical methods adopted by major regulatory authorities throughout the world
- Detailed statistical analysis by country, age, gender, etc.
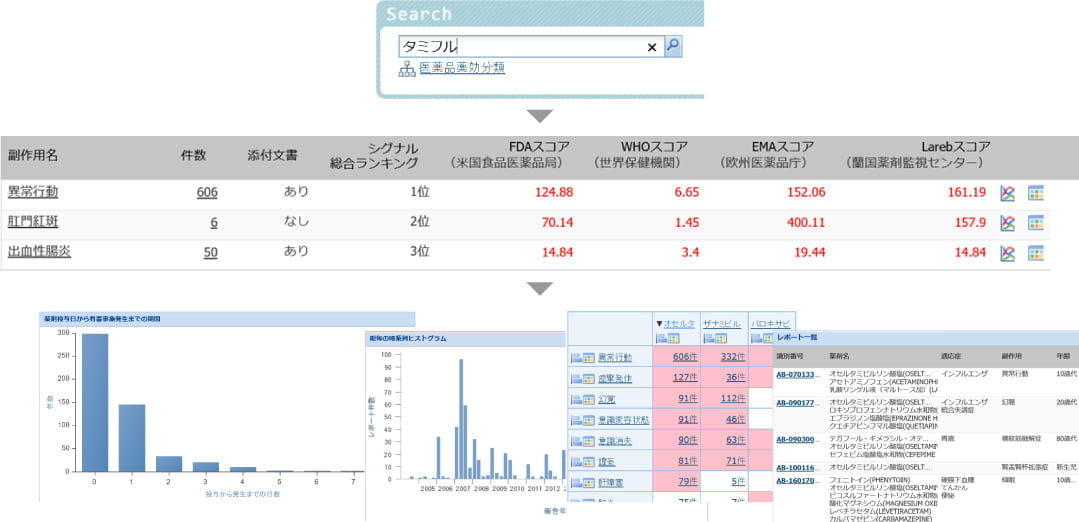
Function 1: Display search/statistical analysis/various graphs
Various search functions and case reports can be displayed.
- Score reports based on search criteria (number of events, score index value)
- Number of days to the onset of event, histogram of the number of reports in each period, etc.
- List of case reports and individual case reports
|image scroll
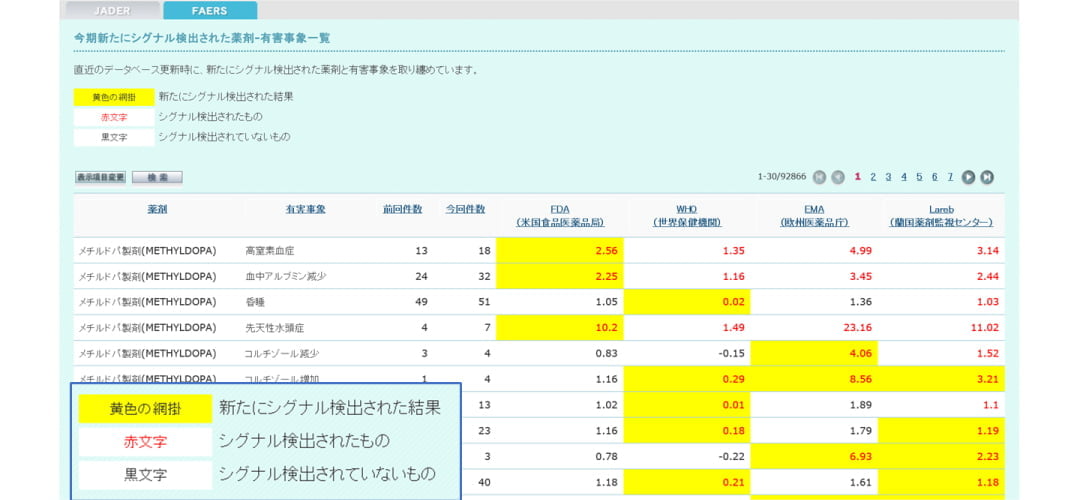
Function 2: Signal detection at the time of database update
Newly detected signal information is extracted and displayed in a table at the time of database update.
You can also sort items and search by drug or adverse event.
Target databases: FAERS (quarterly), JADER (monthly)
|image scroll
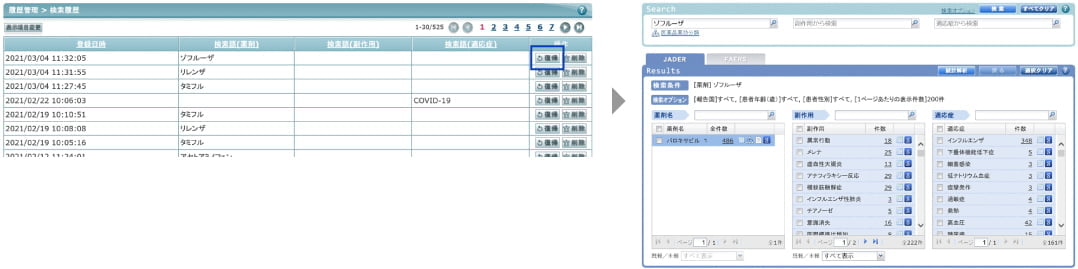
Function 3: Retain the history of search criteria
As the past search criteria are retained as history, it is easy to search with the same criteria.
|image scroll
Signal Detection Support System CzeekR

Installed Databases
- Installed both FAERS and JADER databases
- Enables search and display in Japanese by regular data arrangement including elimination of duplicates at the time of data update.
- Your own database can be installed.
*FAERS (FDA’s Adverse Event Reporting System): a public database administrated by the FDA containing adverse event reports, medication error reports and product quality complaints resulting
*JADER (Japanese Adverse Drug Event Report database): a public database administrated by the PMDA containing adverse event reports.
Signal Calculation System
With a computational function, score values can be derived according to your own data setting.
- Customers can design their own search criteria settings such as database and drugs to calculate score value as a signal detection index by batch processing.
- You can add duplicates elimination criteria and conditions other than country, age and gender.
- Scores are calculated by four statistical methods (PRR, ROR, BCPNN and GPS) adopted by major regulatory authorities throughout the world
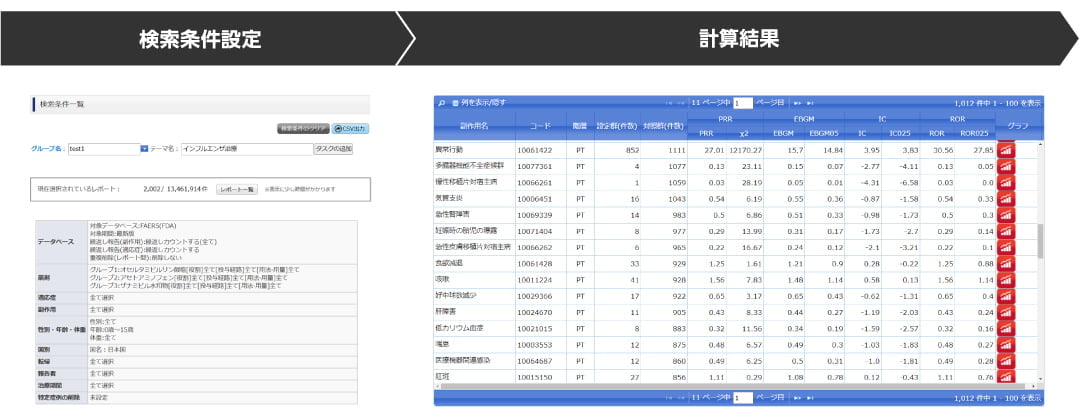
Function 1: Search criteria setting and calculation of signal index values under your own criteria
- Set various conditions from the list of search criteria and calculate the signal detection index value
|image scroll
Function 2: Display various graphs and maps
- Statistical analysis data including signal index values can be checked visually
(- display in chronological order, histogram, heat map, distribution map, etc.)
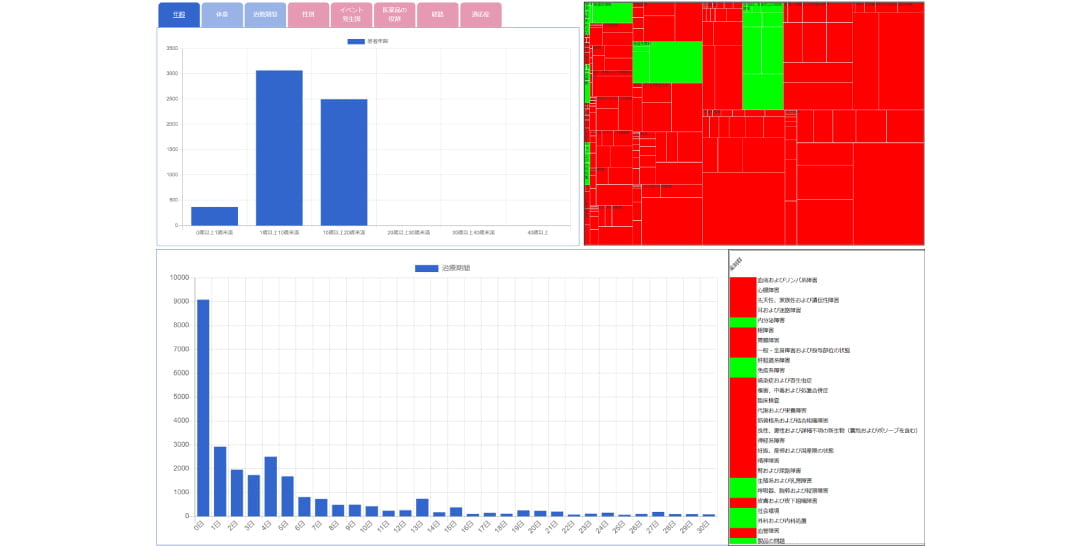
|image scroll
For further information onArk Medical Solutions Inc.
If you have any questions or comments, please do not hesitate to contact us.
Contact UsArk Medical Solutions’s Scope of Service
-
Clinical Development Support Services
From OTC products to ethical drugs and medical devices, we provide complete coverage of the healthcare market
- Monitoring
- EDC-enabled Data Management
- Biostatistics
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
PMS Support Services
We provide various services to respond to the various PMS issues facing our clients.
- Patient Registration
- EDC-enabled Data Management
- Biostatistics
- Auditing/Self-inspection
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
DB Research/DB Survey
A wealth of experience in using real-world databases
Comprehensive support for database surveys/database research by highly professional staffPharmacovigilance (GVP)
Providing speedy and reliable pharmacovigilance service through our well-established team of highly skilled professionals in GVP operations.
-
ADDIN Series
ADDIN series, our EDC system which prioritize operability for physicians who are in charge of post-marketing surveillance, assures efficient and high quality data accumulation/progress management at your survey project.

-
ePRO (Patient Reported Outcomes System)
We provide optimal ePRO system for collection of patient outcome data.
-
Signal Management Service (Adverse Events Information Data Service)
We provide statistical analysis services for adverse event database systems and real-world data of various drugs by utilizing "FAERS" and "JADER," spontaneous adverse event databases collected/published by FDA and PMDA.


