ADDIN Series
ADDIN- Unique PMS-EDC system developed in-house by CRO -
"ADDIN" is a unique EDC system for PMS developed in-house by CRO.
It provides One-stop Support System shared by both CRO and vendors, with a rapid collection function for adverse drug events and PV related information.
With 20 years of evolutionary history with "ADDIN" and "ADDIN EX", current “ADDIN SMALLT” is highly evaluated by doctors and outsourcers with its easy-to-use operability and workflow suitable for PMS.
Service Features
- Point 1.
- EDC system for PMS developed in-house by CRO
- Point 2.
- User-friendly EDC screen easy to operate for Medical Doctors
- Point 3.
- Extensive experience and achievements of consigned projects

|image scroll
Main Services with Details
Basic functions/features
Wide Variety of Data Interactions
ADDIN has the following data management functions to manage large amounts of data management for PMS
- Coding (disease, drug name, adverse event)
- Logical Check (Secondary logical)
- Reexamination Request Form preparation
- Data Management Task Control (including progress follow-up function)
Email Transmission Function
The messaging function in ADDIN supports smooth and reliable delivery of survey/resurvey requests via email.
Record Tracking Functions
(Audit Trail Functions)
ADDIN manages data from initial data entry by medical doctors to the final lock. The recorded data is searchable for confirmation and output.
Survey Specification Changes and Version Upgrades
Changes in survey items and logical check specifications often occur during PMS; however, such specifications and content changes are possible without suspension of the survey.
Electronic Signature Function
The messaging function in ADDIN supports smooth and reliable delivery of survey/resurvey requests by emails.
Optional functions/features
Linkage to external interface
- Progress Follow-up System (ADDIN.SCOPE, POSTIER, PostMa Watch, ClipCapture)
- Safety Database system (Argus, ARISj, ClinicalWorks, Perceive) *EtoB connection
- ePRO system
Capture PDF output
- Convert the screen image of the survey form to PDF for output
Batch logical function
In case the reexamination criteria are revised during operation, received data can be checked with the latest reconciliation standards.
One-stop Support System shared by both CRO and vendors
Smooth, stress-free operation throughout the processes from EDC setup to PMDA application
|image scroll
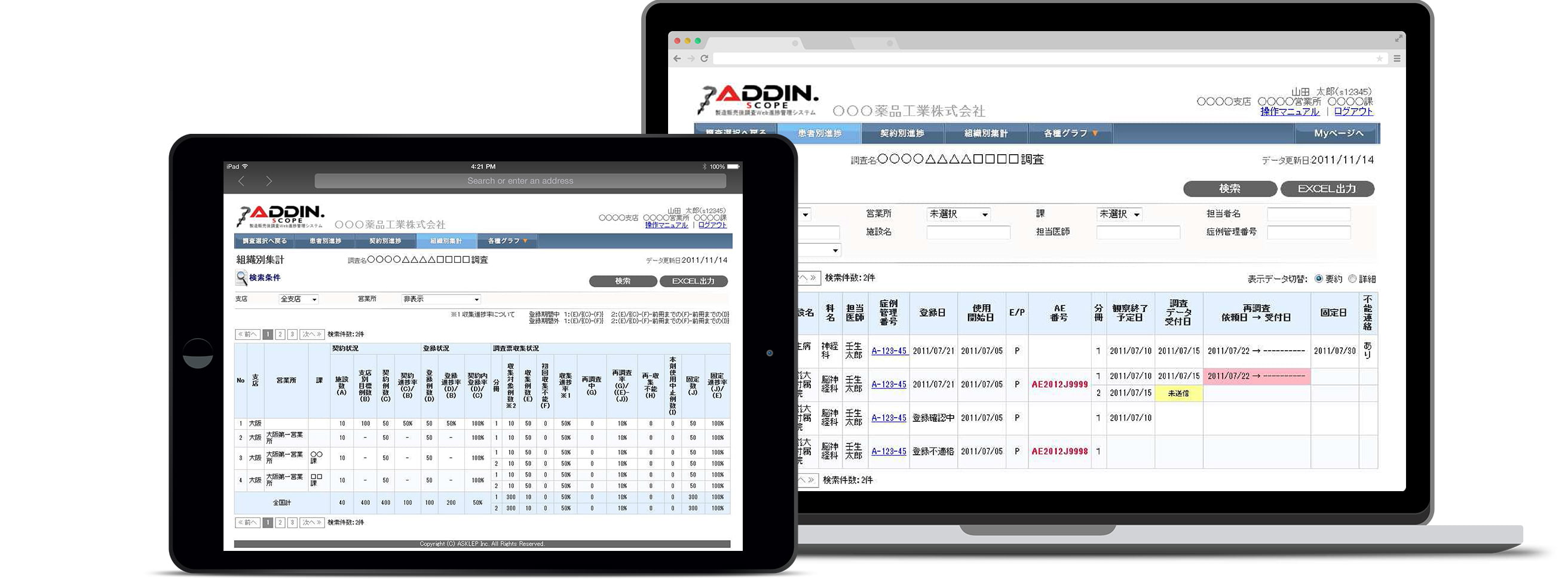
ADDIN.SCOPE- Progress Follow-up System -
Smooth implementation of Post-marketing Surveillance with our PMS platform
ADDIN.SCOPE is our web-based progress follow-up systems available via the internet. Using our system as your PMS platform provides smooth and reliable implementation of surveys from its ability for diverse data interactions. We offer a system best suited to satisfy the needs of our customers.
With ADDIN.SCOPE, the progress transition and passing ratio are checked by the department and visualized from the beginning of a survey.

|image scroll
Service Features
- Point 1.
- Cloud-type, web-based progress follow-up system with very little investment
- Point 2.
- Diverse data interactions and less stressful progress follow-up from the most effective use of EDC / CDMS data
- Point 3.
- User-friendly screens designed with simplicity. Quick and easy information sharing function is also available.
Diverse Data Interactions
- ADDIN series, CDMS, Other EDC system
- Data output from CSV files and progress data imports are conducted every day.
Optional Functions
- My Page
- Contracts information entry
- Fees management
- Tallying alert by department
Standard Functions
- Progress follow-up by patient, contract, and department
- Graph display (progress by month and department)
- Excel output
- Contract information interface
- EDC (ADDIN) survey information interface
- In-house and external EDC survey information interface
- Paper-based survey information interface
- Paper-based survey and CRF tracking (including receiving date)
- Multiple CRO capability (direct data uploads from CROs)
For further information onArk Medical Solutions Inc.
If you have any questions or comments, please do not hesitate to contact us.
Contact UsArk Medical Solutions’s Scope of Service
-
Clinical Development Support Services
From OTC products to ethical drugs and medical devices, we provide complete coverage of the healthcare market
- Monitoring
- EDC-enabled Data Management
- Biostatistics
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
PMS Support Services
We provide various services to respond to the various PMS issues facing our clients.
- Patient Registration
- EDC-enabled Data Management
- Biostatistics
- Auditing/Self-inspection
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
DB Research/DB Survey
A wealth of experience in using real-world databases
Comprehensive support for database surveys/database research by highly professional staffPharmacovigilance (GVP)
Providing speedy and reliable pharmacovigilance service through our well-established team of highly skilled professionals in GVP operations.
-
ADDIN Series
ADDIN series, our EDC system which prioritize operability for physicians who are in charge of post-marketing surveillance, assures efficient and high quality data accumulation/progress management at your survey project.

-
ePRO (Patient Reported Outcomes System)
We provide optimal ePRO system for collection of patient outcome data.
-
Signal Management Service (Adverse Events Information Data Service)
We provide statistical analysis services for adverse event database systems and real-world data of various drugs by utilizing "FAERS" and "JADER," spontaneous adverse event databases collected/published by FDA and PMDA.


