Data analysis Outsourcing Service
Signal Score Report
Signals are calculated according to various settings and provided as a score report in CSV file format. All reported adverse events in regard to the selected drugs are extracted, and the number of cases reported, the number of adverse events, various score values, etc. are summarized in a table.
More criteria, include drug(s) selection, suspected drug setting, specifications setting such as period setting, and other detailed conditions such as “collectively count all target adverse events” and “generate calculation results using SMQ” can be set. The service can be rendered in a single delivery or on a regular basis at the time of every database update. Please feel free to consult us about detailed conditions and circumstances.
[Target databases]
JADER, FAERS, VAERS, CAERS, etc.
*JADER (Japanese Adverse Drug Event Report database): a public database administrated by the PMDA containing adverse event reports.
*FAERS (FDA’s Adverse Event Reporting System): a public database administrated by the FDA containing adverse event reports, medication error reports and product quality complaints resulting
*CAERS (CFSAN Adverse Event Reporting System): a public database administrated by the FDA containing information such as adverse events and product complaint reports for foods, dietary supplements and cosmetics
*VAERS (Vaccine Adverse Event Reporting System): a public database administrated by the FDA containing post-vaccination adverse event report information
Analysis support
In addition to providing a signal score report, we perform contracted analysis using SRS data and other data.
We will examine your desired conditions, such as index, and proceed with analysis.
Please feel free to consult us concerning data analyses using RWD other than SRS data as well.
[Target databases]
JADER, FAERS, VAERS, CAERS, etc.
*JADER (Japanese Adverse Drug Event Report database): a public database administrated by the PMDA containing adverse event reports.
*FAERS (FDA’s Adverse Event Reporting System): a public database administrated by the FDA containing adverse event reports, medication error reports and product quality complaints resulting
*CAERS (CFSAN Adverse Event Reporting System): a public database administrated by the FDA containing information such as adverse events and product complaint reports for foods, dietary supplements and cosmetics
*VAERS (Vaccine Adverse Event Reporting System): a public database administrated by the FDA containing post-vaccination adverse event report information
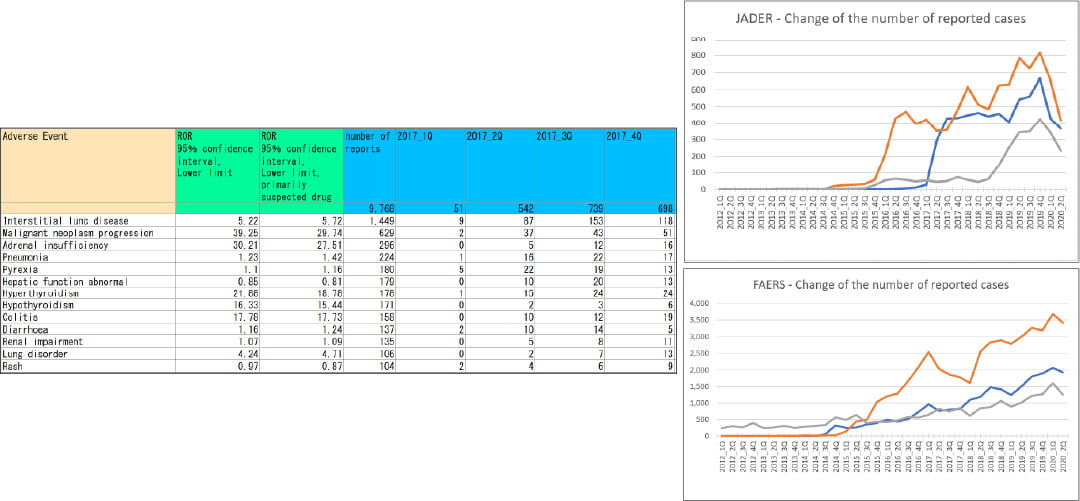
[Analysis case: Changes in the number of spontaneous reports]
- Objective: To grasp the changes before and after the COVID-19 outbreak
- Database: FAERS/JADER
- Results: Signal indicators and the number of adverse events were calculated.
|image scroll
Joint research/independent research
<2017>
- “Unknown/known classification of spontaneous reports of adverse events by comprehensive mapping technology of adverse drug reaction terms”
The 20th Annual Meeting of the Japanese Society of Drug Informatics - “Construction of automatic classification system for unknown/known of spontaneous reports of adverse events by comprehensive mapping of adverse drug reaction terms and its verification by SGLT2”
The 27th Annual Meeting of the Japanese Society of Pharmaceutical Health Care and Sciences - “Integration of the spontaneous reporting database of adverse events, adverse drug reactions in package inserts and RMP safety specifications, and development of the periodic signal monitoring system"
The 23rd Annual Meeting of the Japanese Society for Pharmacoepidemiology
<2018>
Joint research) Comparison of the risk of acute pancreatitis between corticosteroids using the FAERS database
The 21st Annual Meeting of the Japanese Society of Drug Informatics
- Verification of the risk of urinary tract infection associated with SGLT2 inhibitors based on the health insurance claim database and spontaneous report database
The 24th Annual Meeting of the Japanese Society for Pharmacoepidemiology - Investigation of the risk of upper respiratory tract infection with DPP-4 inhibitors using medical databases (health insurance claims/spontaneous reports)
The 28th Annual Meeting of the Japanese Society of Pharmaceutical Health Care and Sciences
<2019>
- Association between diabetes treatment and urinary tract infections: An analysis using data from the JMDC claims databaseJoint hosting with the 25th Annual Meeting of the Japanese Society for Pharmacoepidemiology and the ISPE’s 12th Asian Conference on Pharmacoepidemiology (ACPE)
For further information onArk Medical Solutions Inc.
If you have any questions or comments, please do not hesitate to contact us.
Contact UsArk Medical Solutions’s Scope of Service
-
Clinical Development Support Services
From OTC products to ethical drugs and medical devices, we provide complete coverage of the healthcare market
- Monitoring
- EDC-enabled Data Management
- Biostatistics
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
PMS Support Services
We provide various services to respond to the various PMS issues facing our clients.
- Patient Registration
- EDC-enabled Data Management
- Biostatistics
- Auditing/Self-inspection
- Medical Writing
- Auditing/Self-inspection
- EDC Services
-
DB Research/DB Survey
A wealth of experience in using real-world databases
Comprehensive support for database surveys/database research by highly professional staffPharmacovigilance (GVP)
Providing speedy and reliable pharmacovigilance service through our well-established team of highly skilled professionals in GVP operations.
-
ADDIN Series
ADDIN series, our EDC system which prioritize operability for physicians who are in charge of post-marketing surveillance, assures efficient and high quality data accumulation/progress management at your survey project.

-
ePRO (Patient Reported Outcomes System)
We provide optimal ePRO system for collection of patient outcome data.
-
Signal Management Service (Adverse Events Information Data Service)
We provide statistical analysis services for adverse event database systems and real-world data of various drugs by utilizing "FAERS" and "JADER," spontaneous adverse event databases collected/published by FDA and PMDA.

